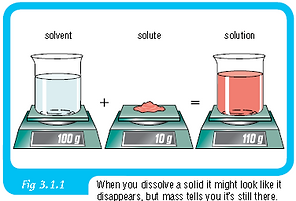
July 15 - July 19
Atoms and Matter Intro
Essential Question: How is matter alike or different at the molecular level?
6.P.2.1: Students will understand: That all matter is made up of atoms, atoms of the same element are alike, and different elements contain different atoms.
Monday
Intro Lesson
What is Matter?
If you zoom into this picture, what do you notice? In your notebook, please write the following:
Matter is composed of extremely small particles called atoms.
These particles are too small to be seen with a microscope.
Atoms have all the properties of matter: mass and volume
Atoms are the smallest part of an element.
All atoms of the same element have the same properties.
Extras
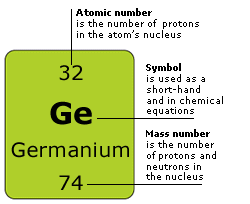
Tuesday - Wednesday
(Work plan, guided lessons)
Vocabulary Foldable


Station 3
Use the resources provided to complete your handout. Create your own illustration and example to demonstrate your understanding.
Station 4
The History of an Atom
-
Summarize the history of the atom.
-
Analyze the evolution of the atomic theory evolve over time. How did it change?
Station 5
Read all about it!
Choose a book from the classroom library to discover more! You can also use these books to research more information for your Element Advertisement you will be completing on Thursday.
Station 6
Happy Atoms!
Are you ready to put your knowledge to the test? Using the app on the iPad and the Happy Atoms kit, find out what happens when you combine atoms together!
Station 7
Write All About It
Write a creative story from the view of an atom. Make sure you use the following vocabulary words: matter, element, atom, mass, volume, molecule.
Thursday - Friday
(Evidence of Learning)
Element Advertisement Major
Using books from the library, students will choose an element to research.
July 22 - July 26
Phase Changes
Essential Question: What happens to the molecules of a substance when heat is added to it?
Objective: 6.P.2.2 Explain the effect of heat on the motion of atoms through a description of what happens to particles during a change in phase.
Monday
Lesson Intro
We will use the first 15 minutes of class to finish our major. Then, we will review!
Matter Review
Let's decompress!
You have learned a lot of information on Atoms and Matter up to this point. Let's take a moment to sit back and review everything you have learned! Find your way through this Prezi!
Tuesday - Wednesday
Balloon Demo/ Work Plan / Guided Lessons
Phases of Matter
Solids, Liquids, and Gases
Phases Changes
-
What do you look like when you have a lot of energy (ex: excited for the weekend)
-
What do you look like when you have some energy (after lunch when you are full)?
-
What do you look like when you have a little bit of energy (after a basketball game or right before you fall asleep at night)

Thursday
Work Plan Check-In
Friday
Evidence of Learning

Checkpoint 1

Happy Track Out!
See you August 19!
August 19 - August 23
STANDARD 1 & 2 REVIEW
Essential Question: How is matter alike or different at the molecular level?
6.P.2.1: Students will understand: That all matter is made up of atoms, atoms of the same element are alike, and different elements contain different atoms.
Essential Question: What happens to the molecules of a substance when heat is added to it?
Objective: 6.P.2.2 Explain the effect of heat on the motion of atoms through a description of what happens to particles during a change in phase.
Monday
Review Week
Let's review from track-out!
What are the parts of an atom?
What is matter?
Review Major
By the end of the quarter, students will show artifacts for 3 Matter Standards. This week we will be adding artifacts to our portfolios and reviewing.
Level 2 Work
Level 4 Work
Complete any of the Level 4 stations from the previous weeks that were not finished.
-
Atoms Story
-
Create Your Own Lab
-
Read, then write!
Level 3 Work
Finish Early?
August 26 - August 30
PHYSICAL PROPERTIES
Essential Question: How are the physical properties used to compare pure substances?
Objective: 6.P.2.3 Compare the physical properties of pure substances that are independent of the amount of matter present during density, melting point, boiling point, and solubility to properties that are dependent on the amount of matter present to include volume, mass, and weight.
Physical Properties Intro
-
What are physical properties of matter?
-
What is the difference between independent and dependent properties?
-
Physical vs Chemical Properties
-
Physical vs Chemical Changes
-
-ELEPHANT TOOTHPASTE!
Tuesday
Solubility Stations


Wednesday/Thursday
Density
Station 1
Mass
-
Find the mass of each object in the bin.
-
Record your findings in your binder.
-
Check your work with the digital scale.
-
Write a How-To for finding the mass of an object.
Station 2
Volume
-
Find the volume of each object in the bin.
-
Record your findings in your binder.
-
How can you find the volume of a regular shaped object?
-
How can you find the volume of an irregular shaped object?
Friday
FINISH STATIONS
Station 3
Finish Early?
Station 4
-
Complete the Density Simulation on the computer.
-
In your binder, answer the following questions –
-
Does the ratio of mass to volume affect the density?
-
What happens when you increase the volume and not the mass?
-
What happens when you increase the mass and not the volume.
-
Using the mystery blocks, list the blocks in order from least dense to most dense.

September 3 - September 6
Physical Properties
Essential Question: How are the physical properties used to compare pure substances?
Objective: 6.P.2.3 Compare the physical properties of pure substances that are independent of the amount of matter present during density, melting point, boiling point, and solubility to properties that are dependent on the amount of matter present to include volume, mass, and weight.
Monday
Wednesday


Element Superhero
Activity
Element Superhero
Activity
Thursday
Friday
Element Superhero
Activity - SHARE OUT
Gummy Bear Lab
We will be reviewing physical properties of matter with a Gummy Bear Lab.

September 9 - September 13
Portfolio Major Review
Essential Question: How are the physical properties used to compare pure substances?
Objective: 6.P.2.3 Compare the physical properties of pure substances that are independent of the amount of matter present during density, melting point, boiling point, and solubility to properties that are dependent on the amount of matter present to include volume, mass, and weight.
Monday - Tuesday
Wednesday
Portfolio Review

Gummy Bear Lab
We will be reviewing physical properties of matter with a Gummy Bear Lab.
Friday
REVIEW
Thursday
Finish Gummy Bear Lab
Review for Major Lab
Want to review on your own? Check out the crash course series reviewing our unit on matter!
September 16 - September 18
Major Lab
Monday - Wednesday
MAJOR LAB
Properties of Matter
Measuring mass, volume
Calculating density
soluble vs insoluble
chemical vs physical reactions
independent vs dependent properties